Published online Sep 28, 2014. doi: 10.4329/wjr.v6.i9.730
Revised: June 20, 2014
Accepted: July 17, 2014
Published online: September 28, 2014
Processing time: 268 Days and 5.5 Hours
The most accurate and practical imaging algorithm for the diagnosis of intestinal malrotation can be a complex and sometimes controversial topic. Since 1900, significant advances have been made in the radiographic assessment of infants and children suspected to have anomalies of intestinal rotation. We describe the current methods of abdominal imaging of malrotation along with their pros and cons. When associated with volvulus, malrotation is a true surgical emergency requiring rapid diagnosis and treatment. We emphasize the importance of close cooperation and communication between radiology and surgery to perform an effective and efficient diagnostic evaluation allowing prompt surgical decision making.
Core tip: Malrotation, especially when associated with midgut volvulus, is a surgical emergency that must be astutely recognized, quickly diagnosed, and emergently treated operatively. While the diagnosis depends heavily on clinical acumen and suspicion, radiologic imaging is critical in determining which patients need surgery. Surgeons and radiologists must cooperate and communicate effectively during the radiographic evaluation of a child with malrotation. Additionally, the algorithm for imaging malrotation must be adapted based upon the tools and staff available at any given institution.
- Citation: Tackett JJ, Muise ED, Cowles RA. Malrotation: Current strategies navigating the radiologic diagnosis of a surgical emergency. World J Radiol 2014; 6(9): 730-736
- URL: https://www.wjgnet.com/1949-8470/full/v6/i9/730.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i9.730
Surgeons are often consulted for evaluation of pediatric abdominal problems presenting to the emergency department. It is common for these patients to be evaluated by radiographic imaging in addition to a focused history and physical examination. The surgeon and radiologist must always have a particularly high-level of suspicion in cases of possible malrotation that may require emergency surgery after evaluation.
A 5-day-old full term male infant presents to the emergency department with continuous bilious non-bloody vomiting and irritability after his last three feeds. He was born by normal spontaneous vaginal delivery without complications and was noted to be breast-feeding well prior to discharge on day-of-life 2; he continued breastfeeding and passing stools at home for the past 4 d until this evening. On exam, his abdomen is minimally distended and he is crying constantly. The clinical picture suggests an obstruction distal to the ampulla of Vater, and the surgeon has a heightened concern for malrotation with midgut volvulus. Before subjecting this infant to the morbidity of surgery, the surgeon calls a colleague in the Radiology Department to discuss appropriate imaging workup for malrotation.
Anomalies of intestinal rotation, commonly referred to as malrotation, are a result of errors during embryologic development. In malrotation, the midgut does not complete its normal lengthening and rotation, and thus is incorrectly positioned within the peritoneal cavity. Normally the process of lengthening and rotation begins between the 4th and 5th wk of gestation. From this time until about week 10, the midgut is outgrowing the abdominal cavity and is forced to herniate through the umbilicus to continue unhindered growth[1]. During weeks 10 and 11, the intestine returns to the peritoneal cavity. From the 11th wk forward, the small bowel undergoes fixation.
The small intestine is a straight tube early in development that derives its blood primarily from the superior mesenteric artery (SMA). This vessel divides the midgut into two parts: the cephlad or prearterial portion, and the caudad or postarterial portion[2]. The prearterial portion is made up of duodenojejunal loops, while the postarterial portion are cecocolic loops[3]. The SMA is important not only because it supplies the majority of blood flow to the small intestine, but also because it serves as the axis for the normal embryologic rotation of the bowel during development.
When the bowel herniates through the umbilicus, the prearterial portion rotates 180° counterclockwise around the axis of the SMA, while the postarterial portion rotates 90° counterclockwise. During the 10th and 11th wk, the prearterial portion of the gut reenters first followed by the postarterial portion. While the bowel returns into the abdominal cavity, both segments complete a total turn of 270°. This configuration places the normal anatomy of the C-loop of the duodenum posterior to the SMA and the transverse colon anterior to the SMA.
The blood from the SMA is distributed throughout smaller vessels running within the mesentery of the bowel. In normal development, the mesenteric root passes along the retroperitoneum from the ligament of Treitz to the proximal cecum[4]. When normal rotation of the small bowel is not completed in embryologic development, the mesenteric root is foreshortened[5]. The small bowel is then supported only by this foreshortened pedicle containing the SMA. The small bowel may then twist (volvulus), about this narrow axis[6]. There are two major types of rotational abnormalities that have been described as malrotation and result in this foreshortening: incomplete rotation and non-rotation[7]. During incomplete rotation, neither the cranial nor the caudal portion rotates more than 180°. The proximal midgut becomes fixed to the right of the SMA and the cecum becomes fixed directly anterior to the SMA. This pattern has the classic features of Ladd’s bands covering and impinging upon the anterior portion of the duodenum and the close proximity of the fixation points for the cranial and caudal midgut along with the SMA. In non-rotation, neither portion rotates more than 90°. Under-rotation leaves the proximal midgut fixed anterior to the right of the SMA and the cecum anterior to the left of the SMA, and the mesentery is still narrowed and foreshortened.
Two individuals recognized for their descriptions of small bowel anatomy and malrotation are Václav Treitz and William Ladd. Treitz (1819-1872), a professor of anatomy in Prague, described the area of tissue which we now recognize as the Ligament of Treitz[3]. This area that bears his name gives physicians a common point to localize where the duodenum becomes the jejunum after exiting the retroperitoneum. Some have described the ligament as a “weak thin membranous structure” that is seldom demonstrated on CT[8].
William Ladd (1880-1967) is considered the father of pediatric surgery in North America. During World War 1, Ladd dedicated his career to the surgical care of children and became surgeon-in-chief at Boston Children’s Hospital[3]. First in 1932 and then again in 1936, he published articles describing his approach to duodenal obstruction and malrotation with midgut volvulus. In these articles, he described a procedure involving detorsion of the volvulized bowel in a counterclockwise fashion, dividing the bands of tissue extending from the cecum across the duodenum and into the lateral peritoneal gutter, and finally spreading the mesentery from the cecum in the left upper quadrant to the small bowel in the right hemi-abdomen. This procedure later became known as Ladd’s procedure[9]. Rather than attempting to restore normal intestinal rotation, Ladd’s operation aimed to convert malrotation to an arrangement of broadened nonrotation with the goal of minimizing the chance of recurrent volvulus[7]. While historically Ladd had the availability of flat plate radiography to guide his work-up of children with malrotation, many different imaging modalities have become available to help guide diagnosis and treatment of this surgical emergency.
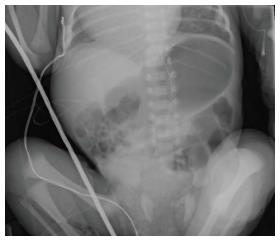
Plain X-ray: Radiographs are often the first step in the imaging evaluation of pediatric patients with suspected malrotation. This relatively inexpensive and widely available test allows the radiologist and surgeon to quickly exclude other potential diagnoses. Unfortunately, the most common finding on plain film of a patient with malrotation is “normal bowel gas pattern”[2]. While abdominal radiographs in a newborn cannot rule-out malrotation, they can occasionally demonstrate findings that are concerning enough to prompt the surgeon to consider operative exploration: “double bubble” sign of duodenal obstruction, lack of bowel gas distal to the duodenum, bowel malposition (small intestine on the right and large intestine on the left, found in non-rotation, Figure 1), or pneumatosis intestinalis with or without portal venous gas.
Ultrasound: Ultrasonography can be used as an adjunct to plain film radiography by determining the position of the superior mesenteric vessels and the relationship to the third portion of the duodenum. In normal anatomy, the SMA lies left of the superior mesenteric vein (SMV); reversal of this relationship may suggest malrotation[10]. Orzech et al[10] state that ultrasound can serve as an excellent screening tool for malrotation especially when complete inversion of the mesenteric vessels along with a “whirlpool” appearance of the mesentery around the SMA is found, prompting urgent exploration. They further suggest that “normal” positioning of the vessels may exist on a spectrum, and thus deviation from the classic position does not always imply malrotation, therefore clinical correlation and pretest probability should direct further studies including possible confirmative upper gastrointestinal studies.
Acknowledging the variation in normal SMA/SMV anatomy, some have supported the use of graded compression ultrasonography as a tool to assess the retroperitoneal position of the third portion of the duodenum (D3). Menten et al[11] state that “based on anatomical and embryological arguments, a retromesenteric D3 excludes intestinal malrotation”. They proposed that the utilization of gradual compression to obtain transverse and sagittal images of the aortomesenteric angle could demonstrate truly normal rotation if D3 was visualized between the aorta and the SMA. Senior pediatric radiologists with over 26 years of combined experience performed the study that affirmed the use of ultrasound over upper gastrointestinal series recommended by Yousefzadeh et al[12] two years earlier based on his own series of pediatric cases.
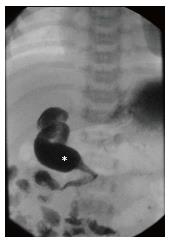
Upper gastrointestinal imaging: Thought of as the “gold standard” test to detect malrotation by most of the pediatric community, upper gastrointestinal imaging series (UGI) utilizes enteral contrast to obtain imaging of the prearterial gastrointestinal tract. An UGI involves administration of contrast orally or into the stomach and capturing images as the contrast traverses the esophagus, stomach, duodenal c-loop, and eventually the duodenal-jejunal junction (DJJ). UGI findings suggestive of malrotation or volvulus include low DJJ position, absence of the DJJ from its typical anatomical position to the left of the vertebral body pedicle, jejunum located on the right (Figure 2), duodenal redundancy, and DJJ corkscrew appearance[13].
Imaging quality depends on the position of the patient during the study, a not insignificant challenge in the pediatric population. In 2013, a group in South Africa published their technique to optimize UGI results[14]. They used external metal markers along the child’s midline to aid in orienting the anatomical position of the patient during the study; they further invested in a three-person team to control the child’s positioning during the entirety of the study. They describe their techniques as follows: “study commences with the child swallowing contrast on their left side (to prevent duodenal filling) to evaluate the esophagus…the child is then placed on its right side to allow duodenal filling and to observe the course of the duodenum…once a sufficient contrast bolus is visualized in the duodenum, the child must be turned rapidly to an unrotated supine position…to capture the c-loop[14]”.
Barium enema: The cecum may be malpositioned in malrotation as is the case with the DJJ; thus, a barium enema can be used to visualize the position of the cecum. Abnormal position of the cecum on preoperative imaging can be found in 80% and 87% of surgically proven cases of malrotation[15]. The normally rotated cecum is found in the right lower quadrant of the abdomen and up to 20% of patients with malrotation will have a normally positioned cecum[2]. No radiographic findings related to the cecum can unequivocally rule-out risk of malrotation[16]. As such, the barium enema is rarely used alone, but may prompt surgical exploration if a patient presents acutely and cecal malpositioning clinically correlates with the patient’s exam.
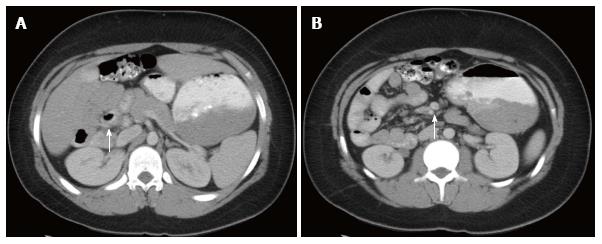
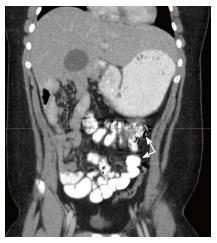
Computed tomography: Like ultrasound, computed tomography (CT) imaging can be used to evaluate the position of D3, the DJJ (Figure 3A), and the anatomical relationship between the SMA and SMV (Figure 3B). Based on a study by Taylor, CT imaging of abnormal D3 position had a sensitivity and specificity of diagnosing malrotation of 97.3% and 99% respectively[17]. Due to the variation in normal SMA/SMV anatomy as previously discussed, the accuracy of identifying “abnormal” SMA/SMV relation in making the diagnosis of malrotation was 76.8%[17]. One unique aspect of a CT is that when used with contrast enhancement it can recognize perfusion abnormalities that may be missed on laboratory studies[18]. CT can be performed quickly on a child with extremely minimal invasiveness, but does subject the child to a significant dose of radiation when compared to an UGI (Figure 4).
MRI: Magnetic resonance imaging (MRI) can be used, much like CT, as a cross-sectional imaging modality to identify findings of malrotation including: dilation of the proximal duodenum, non-retroperitoneal positioning of the duodenum, bowel malpositioning, and inversion of the SMA/SMV relationship[2]. The MRI avoids radiation but relies on the patient holding still for the duration of the lengthier exam. Additionally, the MRI is the most expensive imaging modality available to aid in diagnosing malrotation.
Some believe that localizing the DJJ with UGI cannot give reliable data to rule-out malrotation. One author touts that ultrasonographic imaging in the hands of an experienced technician may demonstrate a retromesenteric D3, which alone can prove that a patient “will not have malrotation and will not develop midgut volvulus[12]”. Menten et al[11] support this assertion, describing a graded compression-technique to demonstrate positioning between the SMA and aorta. These techniques rely on availability of experienced radiology staff, and some hospitals may not have this capability or around-the-clock availability to allow for this focused ultrasound exam. Furthermore, at least one case of normal D3 retroperitoneal positioning on cross-sectional CT imaging in a child with malrotation has been reported, thus calling to question the conclusion that normal positioning always rules out malrotation[17].
One group of infants in particular has added controversy to the approach of workup for malrotation: infants with heterotaxy (Figure 5). Anomalies of intestinal rotation are common in these infants; unfortunately, these children can also suffer from life-threatening cardiac anomalies. There is debate whether these children should undergo elective surgery to broaden the mesentery and prevent volvulus even if an anomaly of rotation is identified[19]. Some have suggested that watchful waiting may be appropriate as volvulus appears to be rare in this population[20]. Importantly, Tashjian et al[21] stated that if a surgeon decides to perform a Ladd’s procedure on a patient with heterotaxia it should occur only when the congenital heart disease is well controlled. Additionally, during operative planning when imaging children with heterotaxia, it is difficult to determine the width of the mesenteric root since there is often insufficient data on the location of the cecum relative to the DJJ[3]. This debate still has yet to be studied in detail with long-term follow-up analysis.
The most important factor in the evaluation of a child with bilious emesis and abdominal tenderness is cooperation between the surgery and radiology teams. Malrotation with volvulus is a surgical emergency in which immediate operative intervention to untwist the volvulus and prevent bowel loss is imperative. Even given prompt diagnosis and preoperative optimization, surgery still carries morbidity and mortality risks associated with anesthesia and the operation itself.
Close communication between the examining surgeon and the radiologist is critical to determine the imaging study best suited to evaluate the given clinical presentation of the child, and to discuss possible limitations of certain studies at specific institutions. Whether initially utilizing radiography/fluoroscopy or cross sectional imaging, lapses in communication should not introduce delay from the time of initial evaluation to the time a decision to operate is made. As discussed, not all imaging results will be straightforward or immediately diagnostic in the evaluation of malrotation, so the coordination of multiple studies should be anticipated and discussed to optimize imaging for the patient.
Knowing the importance of cooperation between the surgeon and the radiologist, we propose below a decision algorithm for an infant or child with possible malrotation. It has been discussed that “negative” radiographic results for most imaging modalities are not 100% reliable in ruling out malrotation. Whenever imaging results are positive for malrotation, we recommend considering operative exploration. It is important to keep in mind that even “positive” radiographic results suggesting malrotation must always be correlated with the clinical picture before committing to an operation.
We suggest beginning with the history and physical along with laboratory results; if there is strong evidence of an emergent ischemic process, the patient may need urgent operative exploration without the delay of imaging. If this is not the case, we recommend starting with easily accessible and inexpensive plain radiography. If the radiograph is negative for evidence of malrotation, the surgeon-radiologist team should discuss whether the hospital is equipped to perform experienced gradual-compression ultrasonography. If there is an experienced radiologist available, the non-irradiating imaging can be performed. If this imaging modality is negative or not available to the team, then an UGI should be performed. Negative UGI results should pause the imaging decision pathway.
Based on the discussed sensitivity and accuracy of the ultrasound and UGI, negative results may strongly suggest that the patient does not have malrotation either my demonstrating normal anatomy or by elucidating evidence of a different diagnosis. At this point, we recommend reassessing the clinical concern for possible malrotation. If the concern is lower, then watchful waiting may be acceptable. If there is still high clinical suspicion, then the imaging should proceed with a barium enema. This pause for decision-making should be short and cooperatively communicated between the surgeon and radiologist, as it would be ideal to obtain a barium enema while the patient is still on the X-ray table from the UGI. A negative barium enema in a patient with high clinical suspicion should prompt a discussion about CT imaging. At this point, do the risks of irradiation with CT imaging outweigh the risks of negative operative exploration or delaying surgery for close observation? If the perceived benefits of the CT outweigh the risks, then CT should be obtained. In the setting of CT results negative for evidence of malrotation, we recommend close observation with low threshold to repeat UGI or consider operation if the exam or labs worsen.
Due to its cost, both in time and dollars, we do not feel that an MRI can give additional information over the previous imaging studies without significantly delaying the diagnosis. Additionally, due to the length of time the patient must remain still, the patient would almost certainly need to be sedated and intubated in order to obtain imaging. We feel the risk of anesthesia for this imaging modality is not acceptable for the benefit of the imaging results gathered.
We recognize that the above algorithm is based on the expectation that plain film radiography, ultrasound, fluoroscopy, and advanced radiography are easily and readily available. Many centers around the world may not have access to these imaging modalities, and the algorithm should be adjusted as such. Additionally, it should be considered that a patient may have intermittent volvulus that, depending on the time of the imaging, may cause false negative results. These cases rely on the clinical evaluation to determine if imaging studies should be repeated.
In conclusion, malrotation presenting in the newborn or older child can become a surgical emergency. Delay in diagnosis, specifically in the setting of a midgut volvulus, can lead to intestinal necrosis, increased mortality, and intestinal failure with dependence on parenteral nutrition. When malrotation is being considered, it is important that pediatric surgeons and pediatric radiologists work closely to discuss available imaging options and communicate a clear workflow of studies while making the decision of whether or not an operation is needed. In the heterotaxy population, even positive imaging can be difficult to interpret clinically and little consensus exists regarding the treatment of this subset of patients.
We have proposed an imaging algorithm based on the current literature and an evaluation of the pros and cons of the different imaging modalities (Table 1). Our algorithm begins with a plain film radiograph followed by either ultrasound or UGI series depending on resources available. However, it is important to stress that any algorithm is useless without the communication and cooperation of the surgery and radiology teams. Teamwork in diagnosis is the key to optimal outcomes in children with malrotation.
| Imaging modality | Pros | Cons |
| Plain film | Inexpensive, quick, may demonstrate classic appearance of duodenal obstruction, may give earlier indication for operative exploration | May masquerade as other abnormalities, may delay treatment (especially when read as “normal”), cannot exclude malrotation |
| Ultrasound | Avoids radiation exposure, may demonstrate “whirlpool sign” indicative of volvulus, duplex to determine relationship of D3 and superior mesenteric vessels, Possibility to evaluate normal abdominal anatomy | Normal sonogram may not exclude malrotation, quality related to technician experience |
| Upper GI | Currently considered the “gold standard”, relatively non-invasive, available at pediatric centers, easily demonstrates duodenal obstruction, allows for visualization of the duodenojejunal junction, delayed imaging may show position of the cecum | Small amount of radiation, challenge to position patient for optimal imaging, may be distorted by bowel distention or indwelling tubes, duodenojejunal junction may have normal variation in position |
| Barium Enema | Easily demonstrates position of entire large bowel (especially cecum) quickly | Small amount of radiation, normal cecum position does not rule out proximal malrotation |
| CT | Quick, allows for viewing position of SMA/SMV, may demonstrate “whirlpool sign” indicative of volvulus, visualization of all abdominal anatomy | High radiation exposure, requires patient to remain still for short period of time, normal relationship between SMA/SMV does not exclude malrotation |
| MRI | No radiation exposure, allows for viewing position of SMA/SMV, may demonstrate “whirlpool sign”, visualization of all abdominal anatomy | Requires patient to remain still for a longer period of time, expensive, not accessible |
We would like to acknowledge Dr. Lauren Ehrlich (Department of Radiology, Yale University School of Medicine) for providing us with de-identified radiographic images.
P- Reviewer: Shen L S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
| 1. | Frazer JE, Robbins RH. On the Factors concerned in causing Rotation of the Intestine in Man. J Anat Physiol. 1915;50:75-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Strouse PJ. Malrotation. Semin Roentgenol. 2008;43:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Lampl B, Levin TL, Berdon WE, Cowles RA. Malrotation and midgut volvulus: a historical review and current controversies in diagnosis and management. Pediatr Radiol. 2009;39:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | McVay MR, Kokoska ER, Jackson RJ, Smith SD. Jack Barney Award. The changing spectrum of intestinal malrotation: diagnosis and management. Am J Surg. 2007;194:712-717; discussion 712-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Daneman A. Malrotation: the balance of evidence. Pediatr Radiol. 2009;39 Suppl 2:S164-S166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Irish MS, Pearl RH, Caty MG, Glick PL. The approach to common abdominal diagnosis in infants and children. Pediatr Clin North Am. 1998;45:729-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 7. | Shew SB. Surgical concerns in malrotation and midgut volvulus. Pediatr Radiol. 2009;39 Suppl 2:S167-S171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Kim SK, Cho CD, Wojtowycz AR. The ligament of Treitz (the suspensory ligament of the duodenum): anatomic and radiographic correlation. Abdom Imaging. 2008;33:395-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Ladd WE. Surgical disease of the alimentary tract in infants. New Eng J Med. 1936;215:705-708. [RCA] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 181] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Orzech N, Navarro OM, Langer JC. Is ultrasonography a good screening test for intestinal malrotation? J Pediatr Surg. 2006;41:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Menten R, Reding R, Godding V, Dumitriu D, Clapuyt P. Sonographic assessment of the retroperitoneal position of the third portion of the duodenum: an indicator of normal intestinal rotation. Pediatr Radiol. 2012;42:941-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Yousefzadeh DK. The position of the duodenojejunal junction: the wrong horse to bet on in diagnosing or excluding malrotation. Pediatr Radiol. 2009;39 Suppl 2:S172-S177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Sizemore AW, Rabbani KZ, Ladd A, Applegate KE. Diagnostic performance of the upper gastrointestinal series in the evaluation of children with clinically suspected malrotation. Pediatr Radiol. 2008;38:518-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Dekker G, Andronikou S, Greyling J, Louw B, Brandt A. Contrast meals and malrotation in children-metal markers for improved accuracy. Pediatr Radiol. 2013;43:115-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Applegate KE. Evidence-based diagnosis of malrotation and volvulus. Pediatr Radiol. 2009;39 Suppl 2:S161-S163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Slovis TL, Strouse PJ. Malrotation: some answers but more questions. Pediatr Radiol. 2009;39:315-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Taylor GA. CT appearance of the duodenum and mesenteric vessels in children with normal and abnormal bowel rotation. Pediatr Radiol. 2011;41:1378-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Aidlen J, Anupindi SA, Jaramillo D, Doody DP. Malrotation with midgut volvulus: CT findings of bowel infarction. Pediatr Radiol. 2005;35:529-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Chang J, Brueckner M, Touloukian RJ. Intestinal rotation and fixation abnormalities in heterotaxia: early detection and management. J Pediatr Surg. 1993;28:1281-1284; discussion 1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Choi M, Borenstein SH, Hornberger L, Langer JC. Heterotaxia syndrome: the role of screening for intestinal rotation abnormalities. Arch Dis Child. 2005;90:813-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Tashjian DB, Weeks B, Brueckner M, Touloukian RJ. Outcomes after a Ladd procedure for intestinal malrotation with heterotaxia. J Pediatr Surg. 2007;42:528-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |